Central Sensitization
Before we begin the discussion about ketamine infusions, the underlying condition that causes many of the painful conditions that are treated with ketamine infusions has to be understood. The Ketamine Infusion Center for Pain (KIC Pain) at the National Pain Centers is one of the largest and most experienced ketamine infusion centers in America. We have had a great deal of success with our customized and individualized protocols that did not happen by accident. Careful and deep understanding of the science is necessary for an advanced program, such as KIC Pain, to be successful. We have performed over 1200 infusions, all under the direct supervision of Dr. Jay Joshi, one of the leading experts on ketamine infusions and central sensitization in the country. Remember, pain is defined as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage", which includes anxiety, depression, CRPS/RSD, fibromyalgia, PTSD, and generalized chronic pain.
What is Central Sensitization?
Central sensitization is a manifestation of activity-dependent plasticity due to an increase in synaptic strength, driven to a substantial extent, by N-methyl-d-aspartic acid glutamatergic receptors. Central sensitization operates after noxious stimuli, peripheral inflammation, and nerve injury in the spinal cord and higher brain centers, and involves multiple presynaptic and postsynaptic changes producing changes in transmitter release and action, as well as synthesis of novel neuromodulators. Many features of central sensitization resemble those that are responsible for memory. Central sensitization is produced not only by increases in excitability but also by a reduction in inhibitory transmission due to reduced synthesis or action of inhibitory transmitters and to a loss of inhibitory interneurons, which may produce a persistent enhancement of pain sensitivity. It has been suggested that central neuronal sensitization plays an important role in postoperative pain.
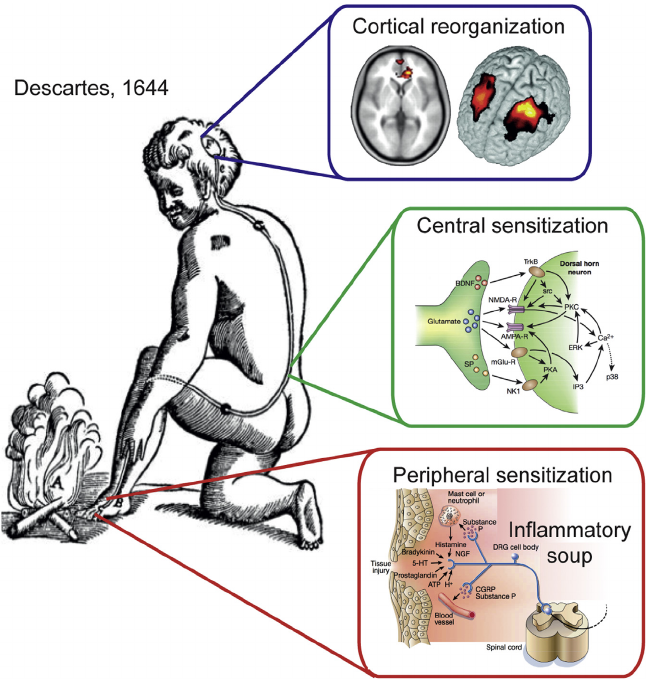
Descartes’ Concept of Sensation Illustrates the Pain System and Its Reorganization Based on Modern Rodent Model Physiology and Human Brain Imaging Studies. Yes, Descartes conceptualized central sensitization almost 400 years ago, yet most people and physicians today still have not even heard of central sensitization.
(A) A stimulus is transmitted to a specific brain region where perception takes place.
(B) System undergoes reorganization following an injury that gives rise to a persistent or chronic pain state.
(C) End-organ injury gives rise to changes locally, collectively described as peripheral sensitization (adapted from Julius and Basbaum, 2001).
(D) Spinal cord circuitry undergoes a large number of changes, resulting in central sensitization (adapted from Scholz and Woolf, 2002), which includes enhanced glutamatergic signaling, changes in second-order messenger processes, and activation of microglia. At the level of the brain, human neuroimaging studies indicate anatomical and functional reorganization.
http://dx.doi.org/10.1016/j.neuron.2015.06.005
What Causes Central Sensitization?
• Potential mechanisms implicated in central sensitization:
• NMDA receptor activation
• Altered gene expression in dorsal horn neurons
• Decreased inhibition
• Microglial activation
• Thalamic and somatosensory cortex changes
Types of Central Sensitization
• Anxiety
• Chronic Pain (In general)
• CRPS/RSD
• Depression
• Fibromyalgia
• Headaches
• Opioid Induced Hyperalgesia
• Phantom Limb Pain
• PTSD
Neurophysiology of Central Sensitization
(A) Transfer of information about the intensity, duration, and location of peripheral noxious stimuli.
(B) Activity-dependent synaptic plasticity driven by high levels of nociceptor input that results in activation of intracellular kinases that phosphorylate ion channels and receptors, altering their distribution and function and increasing excitability and thereby pain sensitivity.
(C) Changes in transcription in dorsal horn neurons. Some alterations in gene expression are activity driven and others are widespread, like the induction of (Cox-2).
(D) Inhibitory interneurons play a major role in damping down sensory processing. After peripheral nerve lesions, there is a reduction in the action of inhibitory transmitters and a loss of γ-aminobutyric acid–mediated interneurons, resulting in a loss of inhibition (disinhibition) producing pain hypersensitivity.
AA = arachidonic acid; AMPA =α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; EP = prostaglandin receptor; IL1β= interleukin 1β; NK1 = neurokinin 1; NMDA = N-methyl-d-aspartic acid; PGE2 = prostaglandin E2; TrkB = tyrosine kinase
Functional MRI (fMRI) Imaging Evidence of Central Sensitization in CRPS
Lenz M, Höffken O, Stude P, Lissek S, Schwenkreis P, Reinersmann A, Frettlöh J, Richter H, Tegenthoff M, Maier C.: Bilateral somatosensory cortex disinhibition in complex regional pain syndrome type I. Neurology. 2011 Sep 13;77(11):1096-101. Epub 2011 Aug 31
Chronic Pain and Central Sensitization
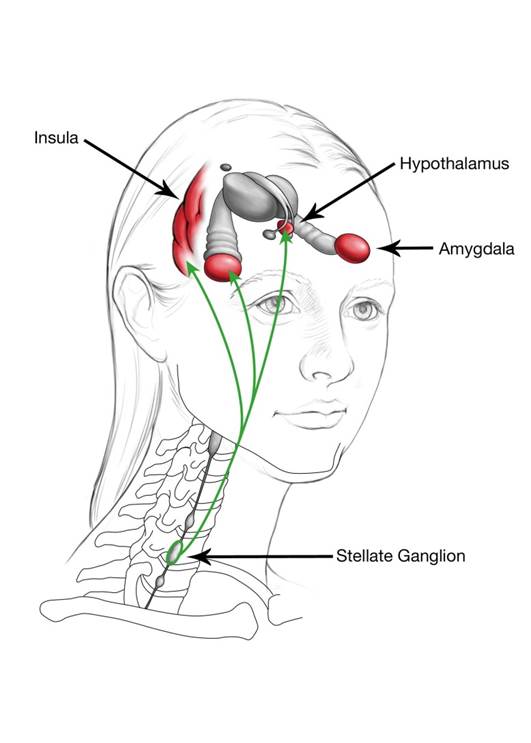
Neural Connection Between the Stellate Ganglion and Central Sensitization
Neural connections between the stellate ganglion and the hypothalamus, amygdala, and regions of the prefrontal cortex, in particular the insular cortex, might explain the effect of stellate ganglion block on Central Pain conditions.
Treatments for Central Sensitization and CRPS
Therapy Based:
• Physical therapy
• Mirror box therapy
• Graded motor imagery
• Tactile discrimination training
• Sensory discrimination training
Neuropsych Based:
• EEG Biofeedback
• Cognitive Behavioral Therapy
• Relaxation Techniques
• Hypnosis
Medications:
• Alpha- or beta-adrenergic-blocking compounds
• Anti-inflammatories (corticosteroids, COX-inhibitors)
• Bisphosphonates
• Botox
• Calcium-regulating drugs
• GABA analogs
• Ketamine
• Local Anesthetics
• Opioids
• SNRIs
• Vasodilators
Interventional:
• Epidural Blockade
• Intravenous immunoglobulin
• Intravenous regional sympathetic block
• Ketamine Infusion
• Selective sympathetic ganglion nerve blocks
• Spinal cord stimulators